
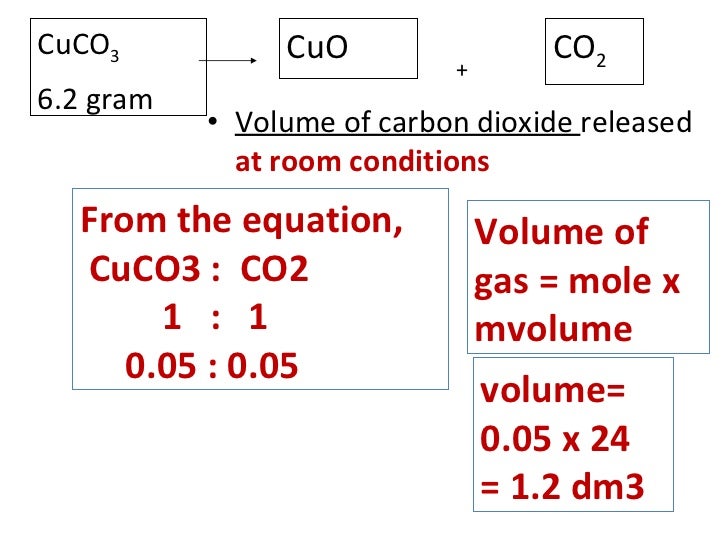
(1).png)
What mass of carbon dioxide, CO2, contains the sam number of molecules as 3.00 g of trichorofluoromethane, CCl3F? How would you solve this? In methanol between single bond In carbon dioxide double bond. What is the difference between a carbon-oxygen bond in your model of methanol and the one in carbon dioxide? (1×12)+(2×16)44 Hence the relative molecular mass of carbon dioxide 44. It is found in the gas state at room temperature, and as the source of available carbon in the carbon cycle, atmospheric CO 2 is the primary carbon source for life on Earth. The combined mass of the products is 21 kg + 85 kg. Molecular formula of carbon dioxide CO 2 The atomic mass of Carbon 12 The atomic mass of Oxygen 16 1× atomic mass of carbon +2× atomic mass of oxygen. Carbon dioxide ( chemical formula CO2) is a chemical compound made up of molecules that each have one carbon atom covalently double bonded to two oxygen atoms. What is the total combined mass of carbon dioxide and water that is produced?Ĭhemical transformation does not change the mass. When the gasoline burns, 85 kg of oxygen is consumed and carbon dioxide and water are produced. What Is The Mass Of 6.9 Moles Of Sulfur Dioxide?Īn automobile gasoline tank holds 21 kg of gasoline. The Mole Ratio Of Butane To Carbon Dioxide In This Reaction is 1:4. a reaction pathway that transforms carbon dioxide (CO 2) into ethanol (C 2 H 6 O). Oxygen is the Necessaries for Living Organism.ĬH4 + 2O2 → CO2 + 2H2O If 1.0 Mole Of Methane Reacts With Oxygen To Produce Carbon Dioxide And Water, What Mass Of Water Is Produced?ĢC4H10 + 13O2 = 8CO2 + 10H2O What Is The Mole Ratio Of Butane To Carbon Dioxide In This Reaction? The molecular formula for ethanol is C2H6O and its molar mass is 46.
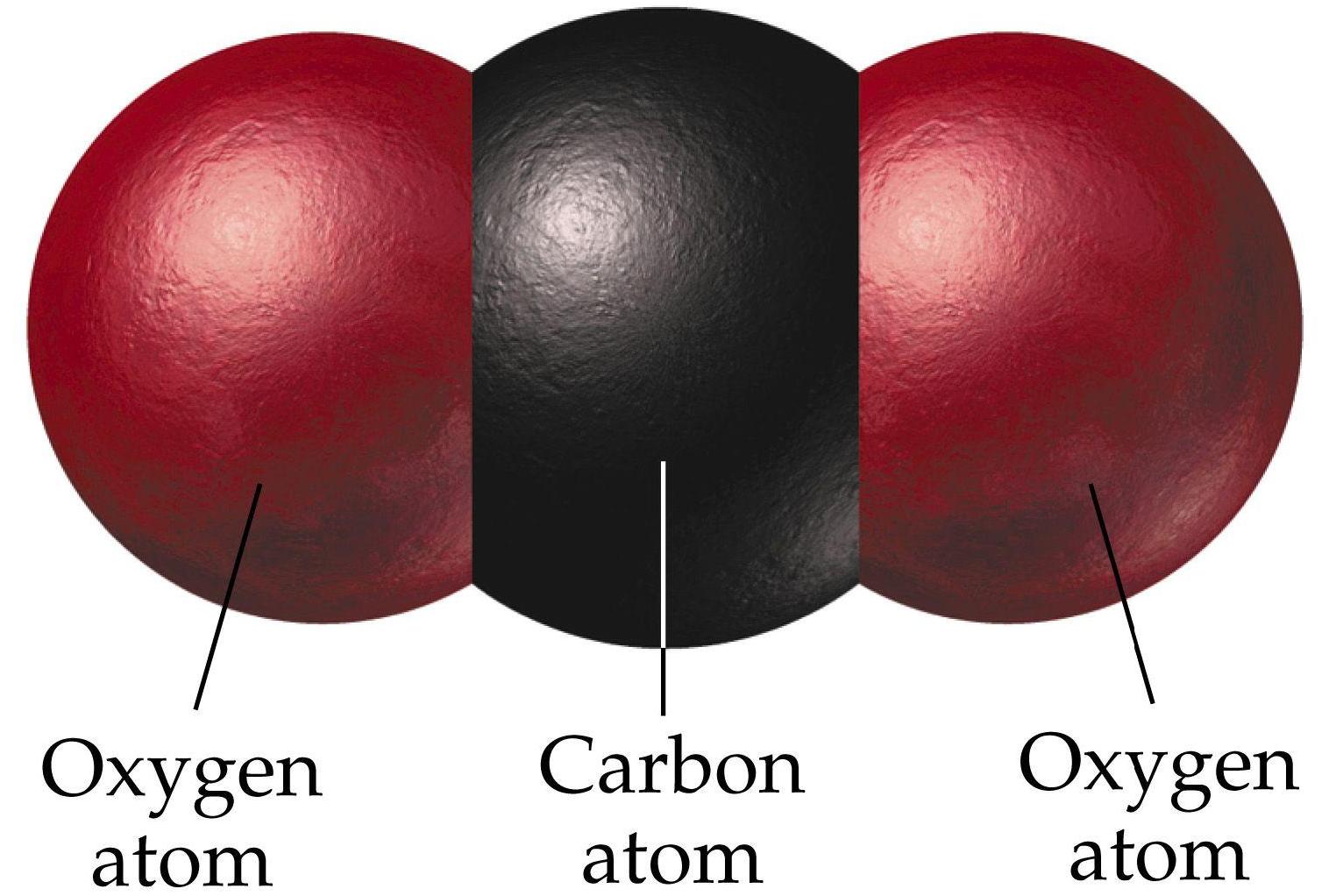
What Is The Importance Of Oxygen And Carbon Dioxide? We know that Oxygen has an atomic mass of 16. What Is The Mass In Grams Of One Mole Of Sulfur Dioxide (SO2)? We see carbon dioxide in soft drinks or beer. Carbon Dioxide is a chemical formula or CO2


 0 kommentar(er)
0 kommentar(er)
